1. Introduction
Microbial polyhydroxyalkanoates (PHA) are a family of biopolyesters synthesized by many bacteria as carbon and energy reserve materials. Poly-R-3-hydroxybutyrate (PHB) was the first PHA family member reported [1]. Thereafter, medium chain-length (mcl) PHA containing 3-hydroxyhexanoate (3HHx), 3-hydroxyoctanoate (3HO), 3-hydroxydecanoate (3HD), and 3-hydroxydodecanoate (3HDD) were found [2]. In 1995, already 91 different hydroxyalkanoic acids had been reported as monomers of PHA [3]. PHA diversity has been further expanded to include microstructures of the PHA polymer chains, such as homopolymers, random and block copolymers, block-random copolymers, functional polymers, graft polymers, thiopolyesters, rare PHAs, as well as their various combinations [4].
PHA are becoming important due to their many advantages. PHA with their unique combination of biodegradability, biocompatibility [5], controllable thermal and mechanical properties [5], [6], [7], as well as PHA molecular weight diversity ranging from several ten thousands to several million Daltons, can be designed and synthesized via controlling the PHA synthase structures and activities as well as the metabolic pathways [8], which is not easily achieved using chemical synthesis approaches [9]. As thermally processible materials, PHA can be used as environmentally friendly plastics for packaging purposes. And their biodegradability and biocompatibility allow them to be used as bioimplant materials for medical and therapeutic applications [5].
Potential therapeutic applications of PHA includes medical implant such as heart valve tissue engineering [10], [11], vascular tissue engineering [12], [13], bone tissue engineering [14], [15], [16], cartilage tissue engineering [17], [18], nerve conduit tissue engineering [19], [20] as well as drug delivery carrier matrix [21], [22]. Many of relevant studies have been conducted in the authors' lab in the past 20 + years [23]. PHA implants were found luckily not to cause carcinogenesis during long-term implantation [24].
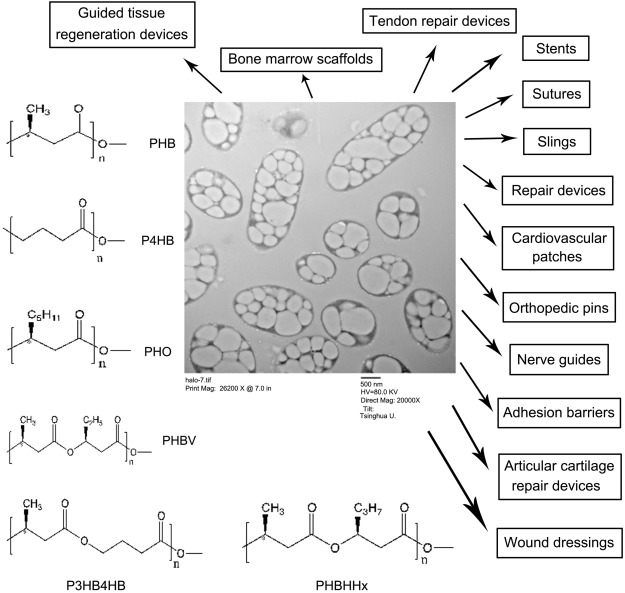
Poly-3-hydroxybutyrate (PHB), copolymers of 3-hydroxybutyrate and 3-hydroxyvalerate (PHBV), poly-4-hydroxybutyrate (P4HB), copolymers of 3-hydroxybutyrate and 3-hydroxyhexanoate (PHBHHx) and poly-3-hydroxyoctanoate (PHO) and its composites have been used to develop sutures, repair devices, repair patches, slings, cardiovascular patches, orthopedic pins, adhesion barriers, stents, guided tissue repair/regeneration devices, articular cartilage repair devices, nerve guides, tendon repair devices, bone marrow scaffolds, and wound dressings [5], [25], [26], [27] (Fig. 1). This paper reviews what have been achieved in the recent PHA tissue therapeutic area and look to the future for PHA as a tissue engineering materials.

Fig. 1. Microbial intracellular polyhydroxyalkanoates (PHA), structures of PHA available in sufficient quantity for biomedical research, and their applications in medicine.
PHB: poly-(R)-3-hydroxybutyrate; P4HB: poly-4-hydroxybutyrate; PHO: poly-3-hydroxyoctanoate; PHBV: random copolymers of (R)-3-hydroxybutyrate and (R)-3-hydroxyvalerate; PHBHHx: random copolymers of (R)-3-hydroxybutyrate and (R)-3-hydroxyhexanoate; P3HB4HB: random copolymers of (R)-3-hydroxybutyrate and 4-hydroxybutyrate.
2. Applications as medical implant materials
2.1. Unique PHA implant forms
2.1.1. 3D printing
3D PHB porous cubes were successfully built with 3D printing technology. The resulting cubes presented shape and dimensions very close to the corresponding virtual model. Compared to clinically used PLGA, PGA and PLA materials, PHB composite implants maintain stable local pH during degradation, which makes them well tolerated by cells and immune system. PHB composites can be used for personalized 3D printed implants [28]. Pre-existing cone-beam computed tomography scans were successfully used for 3D printing of custom-made scaffolds prepared from tricalcium phosphate-PHB (TCP-PHB) according to the individual geometry of the alveolar bone in patients with clefts [29]. The scaffolds were seeded with commercially available human mesenchymal stem cells (hMSCs). The successful osteogenic differentiation of the scaffold-seeded cells could be demonstrated. The 3D implants made of P3HB or P3HB/HA facilitate reconstructive osteogenesis and show pronounced osteoplastic properties. Their in vivo degradation is slow and corresponds to the growth of a new bone tissue, facilitating normal reparative osteogenesis [30]. The ability of porous 3D implants P(3HB) to favor attachment and facilitate proliferation and directed differentiation of mesenchymal stem cells (MSCs) was studied in the culture of MSCs isolated from bone marrow and adipose tissue. And results suggest that P(3HB) has good potential as osteoplastic material for reconstructive osteogenesis [31].
2.1.2. Lithography
Soft lithographic methods including micro-molding and hot embossing were established to produce PHBHHx arrays of microstructures for hosting and culturing cells in a local microenvironment in controlled shapes [32]. Siliconmasters with high-aspect-ratio microfeatures were fabricated using KOH and DRIE anisotropic etching to create PHBHHx microstructures with different configurations including circles, rectangles. PHBHHx microstructures created to mimic cellular microenvironment provide a convenient way to understand relationships of microstructures and cell functions [32].
2.1.3. Nanofibers
3D-nanofiber matrices based on PHA polymers were prepared to mimic the real microenvironment of extracellular matrix (ECM) for cell growth [33]. They have average fiber diameters of 50–500 nm, very similar to the major ECM component collagen. Mechanical properties of PHB/PHBHHx and PHB/P3HB4HB nanofiber matrices were significantly improved compared to PLA ones. Growth of human keratinocyte cell line HaCat on the nanofiber PHA matrices showed an enhanced improvement over those on PHA matrices prepared via an ordinary solution casting method [33]. None of the fibrous scaffolds produced by electrospinning from PHAs had any adverse effects on attachment, growth, and viability of NIH 3T3 mouse fibroblast cells, and all of them were found to be suitable for tissue engineering applications [34].
2.1.4. Injectables
PHBHHx dissolved in not harmful organic solvents including N-methyl pyrrolidone (NMP), dimethylacetamide (DMAQ), 1,4-dioxane (DIOX), dimethyl sulfoxide (DMSO) and 1,4-butanolide (BL), respectively, as an injectable implant can immediately forms a film around the injection site of an animal. One of the applications of PHBHHx injectable implant could be a tissue adhesion prevention film for surgical operations [35].
2.2. Bone tissue engineering
PHA have been demonstrated to support bone tissue growth in various degrees: PHBHHx had the best performance compared with PLA and PHB on attachment and proliferation of rabbit bone marrow cells and thus bone tissue regenerations [36], [37]. PHB blended with hydroxyapatite (HAP) led to improvements on compressive elastic modulus, maximum stress and osteoblast responses including cell growth and alkaline phosphatase activity both in vitroand in vivo [38], [39], [40], [41], [42], [43]. The presence of nanobioglass in the PHB scaffolds significantly improved osteoblast-like MG63 cell proliferationcompared to that of the pure PHB scaffolds and controls [44].
When coated with collagen I and collagen I/chondroitin sulfate, PHB scaffolds seeded with hMSCs guided osteogenical differentiation [45]. When implanted into nude rats, blood vessels were found in the scaffold-stack together with osteogenic markers such as osteopontin, osteonectin, and collagen I around the PHB fibers, demonstrating a successful in vivo osteogenic differentiation and growth [45]. PHBV scaffolds were also successfully used to repair spinal cord injury [46]. PHBHHx/collagen scaffolds were successfully used to culture hMSC over an extended 20 days period supporting the potential of this scaffold combination in future bone tissue engineering applications [47].
When PHA granule binding protein PhaP fused with RGD peptide (PhaP-RGD) was coated on PHBHHx scaffolds, more homogeneous spread of human bone marrow mesenchymal stem cells (hBMSCs), better cell adhesion, proliferation and chondrogenic differentiation in the scaffolds were observed compared with those of PhaP coated or uncoated scaffolds immerging in serum minus chondrogenic induction medium, suggesting that PhaP-RGD coated PHBHHx scaffold promoted chondrogenic differentiation of hBMSCs and could support cartilage tissue engineering [48].
PHBHHx/PHB porous scaffolds with improved mechanical property and biocompatibility were prepared for the three-dimensional growth of chondrocytes isolated from rabbit articular cartilage (RAC). Chondrocytes proliferated better on and in the PHBHHx/PHB scaffolds than on and in the PHB one [49]. PHBHHx has a positive effect on extracellular matrix production. Total collagen contents in all scaffolds containing PHBHHx increased with cultivation time, demonstrating that the presence of PHBHHx in PHB highly favored the production of extracellular matrix of articular cartilage chondrocytes both in vitro [50] and in vivo [51].
PHBHHx was observed to fix soft tissue such as tendon injuries and defects in a rat Achilles tendon repair model. PHBHHx scaffolds showed comparable mechanical properties to rat tendon. No secondary immune response to PHBHHx was observed over a 40 days period of implantation. Movement was restored in PHBHHx scaffold-collagen-tenocyte recipient rats most early than in other experimental groups, demonstrating PHBHHx scaffolds useful for in vivotendon repairs [52].
Strong and elastic PHBHHx was studied for application as a tarsal substitute by implanting it into tarsal defects of Sprague-Dawley rats. Both PHBHHx scaffolds and commercial acellular dermal matrices (ADM) provided satisfactory repair results compared with the blank controls even though the implanted PHBHHx scaffolds showed two weeks light inflammation [53].
CO2 laser radiation was used to process PHB constructs – films and 3D pressed plates. Pulsed laser processing of the 3D plates produced perforated scaffolds with improved mechanical properties and high biocompatibility with bone marrow-derived multipotent, mesenchymal stem cells, which show great promise for bone regeneration [54].
2.3. Artificial blood vessels and heart valve
P3HB4HB has highly adjustable elasticity and strength, it can induce elastinformation, promising for developing into a material for constructing artificial blood vessels [55]. Much stronger block polyurethanes based on P3HB4HB-diol and poly (propylene glycol)-poly(ethylene glycol)-poly(propylene glycol) (PPG-PEG-PPG) were synthesized using 1,6-hexamethylene diisocyanate (HDI) as end-capped agent [56]. The block copolymers had a lower platelet adhesion than the raw materials and the amount of platelet adhesion could be controlled by varying the segmental length of P3/4HB-diol, suggesting that these polyurethanes be candidates as a blood vessel material [56]. A blood vessel material based on composite materials of bacterial cellulose and PHBHV was also reported [57]. So far, PHA based blood vessel materials have been developed with the required bio- and mechanical properties, yet the in vivo test on their suitability has not yet completed.
Hybrid valves fabricated from decellularized porcine aortic valves coated with PHBHHx were implanted in pulmonary position in sheep without cardiopulmonary bypass. The hybrid valve conduits maintained original shapes covered by a confluent layer of cells with less calcification than uncoated control after 16 weeks [11]. The mechanical test in vitro revealed that PHBHHx coating improved tensile strength. PHBHHx coating reduced in vivo calcification and promoted the repopulation of hybrid valve with cells of the recipient resembling native valve tissue. The hybrid valve may be a suitable valve replacement [11]. Tissue-engineered heart valve scaffolds fabricated from P4HB was also reported successfully for implantation in the pulmonary position with an appropriate function for 120 days in lambs [10].
2.4. Artificial nerve conduits
PHB scaffold promoted attachment, proliferation and survival of adult Schwarm cells and supports marked axonal regeneration within the graft [58]. PHB conduits were also found to support in a rabbit common-peroneal-nerve injury model for peripheral nerve regeneration [59]. More elastic PHBHHx nerve conduits was successfully developed into porous PHBHHx conduits for repairing in vivo nerve damage [60].
Xu et al. compared PHB, P3HB4HB and PHBHHx regarding their ability to supported central nervous system (NSC) growth and differentiation both on their 2D films and 3D matrices [61]. PHBHHx one showed the strongest potentials to promote NSC differentiation into neurons which is beneficial for CNS repair. Compared to the 2D films, 3D nanofiber matrices appeared to be more suitable for NSC attachment, synaptic outgrowth and synaptogenesis. PHBHHx nanofiber scaffolds (matrices) were more suitable for treating central nervous system injury.
A more flexible terpolyester of 3-hydroxybutyrate, 3-hydroxyvalerate and 3-hydroxyhexanoate, abbreviated as PHBVHHx, was employed to compare with PLA, PHBHHx for their respective functions leading to more extensive differentiation of human bone marrow mesenchymal stem cell (hBMSC) into nerve cells [62].
PHA blend films with varying ratios of poly(3-hydroxyoctanoate)/PHB abbreviated as PHO/PHB, were found demonstrating higher roughness compared with the neat films and increase in the stiffness when the proportion of PHB increased [19]. The 25:75 PHO/PHB blend showed significantly stronger support for growth and differentiation of neuron cells.
3. Applications as drug delivery matrices
Hydrophobic micro- or nanoparticles PHA are suitable for carrying hydrophobic drugs [63]. A specific drug delivery system consisting of PHA nanoparticles, PhaP and polypeptide or protein ligands fused to PhaP was developed [64]. The ligand-PhaP-PHA specific drug delivery system was proven effective both in vitro and in vivo for targeting cancer cells [64]. Similarly, The specificity of the PHB-PhaC-GFP-A33scFv nanoparticle towards the colon cancer cell lines SW1222 (A33 +) and HT29 (A33-) was confirmed when PHB nanoparticles were attached with engineered PHA synthase fused with green fluorescent protein (GFP) and a single chain variable fragment antibody (A33scFv) specific to colon cancer [65].
Hydrophobic PHA can also be modified to encapsulate hydrophilic drugs. Hydrophilic insulin blending with phospholipid was successfully loaded on PHBHHx nanoparticles (INS-PLC-NPs). Only 20% encapsulated insulin was released in vitro within 31 days. The hypoglycaemic effect in STZ induced diabetic rats lasted for more than 3 days after the subcutaneous injection of INS-PLC-NPs, significantly prolonging the therapeutic effect compared with the administration of insulin solution [66]. PHB-PEG nanoparticles can also achieve insulin encapsulation, cell uptakes and sustainable releases [67], [68].
Adenoviruses carrying a green fluorescence protein gene were complexed with PHBHHx-microspheres having the diameter of capillary (510 μm) after 30 min of co-incubation. The complexes were then injected into the pancreas of mice via the celiac trunk. Approximately 40% of endothelial cells in the pancreas were labeled 5 days after surgery. Islet cells were labeled occasionally, whereas labeling of the acinar and ductal tissues was barely detectable. Efficient pancreatic capillary targeting by using PHBHHx microsphere-adenoviral complexes was established for the treatment of capillary-related diseases [69].
Slow releases of toxic anticancer drugs are an important area of drug delivery. A sustained release system of phosphoinositide-3-kinases (PI3K) inhibitor (TGX221) based on PHBHHx nanoparticles (NP) was developed for blocking proliferation of cancer cell lines [70]. TGX221 was gradually released from PHA-based NP and growth of cancer cell lines was significantly slower in NP-TGX221-treated cells than in either negative controls or in cells receiving free TGX221. A suppression effect by the cisplatin loaded poly(4-hydroxybutyrate)-mPEG (P(4HB)-mPEG) nanocarriers on HT22 cancer cell growth, and enhancement of apoptotic process of the cells compared to free drug treated cells was clearly observed, demonstrating the effectiveness of the amorphousP4HB nanocarriers for the sustained delivery of toxic anticancer drugs [71].
PHB, PHBHHx and PLA nanoparticles encapsulating over 75% of the lipid-soluble colorant rhodamine B isothiocyanate (RBITC) could be used effectively to achieve intracellular controlled drug releases, respectively [72]. Macrophage endocytosis led to an intracellular RBITC drug sustained release over a period of at least 20 days for PHB and PHBHHx nanoparticles, compared with only 15 days and a week for PLA nanoparticles and free drug, respectively.
The diameter of microparticles based on PHA is most significant affected by the concentration of the polymer solution and the method of emulsion mixing. The absence of sharp surges of the drug into the medium and the low rates of drug release from microparticles based on PHB and PHB–PHV copolymers make it possible to state that microparticles are useful for the deposition of drugs. The rate of drug release from microparticles in vitro into the medium is higher in the case of PHB–PHV copolymers than in the case of the PHB [73].
Fibroblast NIH 3T3 cells were cultivated on PHA microparticles, and results showed that microparticles prepared from PHAs of different chemical compositions did not exhibit cytotoxicity to cells cultured on them and proved to be highly biocompatible. Cell attachment and proliferation on PHA microparticles were similar to those on polystyrene. The cytostatic drug encapsulated in P3HB/3 HV microparticles has been proven to be effective against HeLa tumor cells [74].
PHA can also be exploited as gene delivery vector. The mPHA-g-bPEI copolymers were synthesized through Michael addition between acrylated monomethoxy-poly(hydroxyalkanoates) (mPHA-acrylated) and branched poly(ethyleneimine) (bPEI). Results showed that mPHA-g-bPEI copolymers could effectively bind siRNA, protecting it from degradation by nucleases and efficiently releasing the complexed siRNA in the presence of low concentrations of polyanionic heparin. Those mPHA-g-bPEI copolymers revealed a higher transfection efficiency and lower cytotoxicity than bPEI in cultured A549-Luc and MCF-7-Luc cells [75].
Rhodamine-B-loaded PHBHHx nanoparticles were prepared using a modified single-emulsion technique, and then the surface of PHBHHx nanoparticles were coated with low concentrations of the 10 kDa branched PEI in order to facilitate nanoparticle binding to and uptake by cells. The simple and safe nanoparticulate system can be used for in vitro and ex-vivo cellular manipulation. The system could further prove attractive for compartmental administration in brain, solid tumors, and synovial joints et al. when simultaneous delivery of therapeutic agents to a wide range of local cells is needed including simultaneous killing of tumor cells, tumor vasculature endothelial cells, and suppressive T cells in a solid tumor compartment [76].
4. Applications of PHA degradation products-the oligomers and monomers
Biological responses to PHA degradation products oligo-hydroxyalkanoates (OHAs) and monomers are critical factors deciding PHA biocompatibility when they are used for implant applications. Oligo (3-hydroxybutyrate) (OHB, Mn 2000), oligo(3-hydroxybutyrate-co-4-hydroxybutyrate) (O3HB4HB, Mn 2100, 6 mol% 4HB), oligo (3-hydroxybutyrate-co-3-hydroxyhexanoate) (OHBHHx, Mn 2800, 12 mol% 3HHx) and medium-chain-length oligo(3-hydroxyalkanoates) (OmclHAs, Mn 2300, 2 mol% 3-hydroxyhexanoate (3HHx), 25 mol% 3-hydroxyoctanoate, 71 mol% 3-hydroxydecanoate and 3 mol% 3-hydroxydodecanoate) encapsulated in liposomes were found to facilitate their transfer into the cytosol, respectively [77]. OHAs in concentration lower than 20 mg/L did not significantly affect L929 cell viability, the cytotoxicity of OHAs decreased with increasing OHAs side chain length. When OHAs over 40 mg/L, they showed reduced cell viability with more apoptosis, more cell death, delayed cell cycle and reduced cell proliferation [77].
Three oligo-hydroxyalkanoates (Oligo-HAs) including oligo(3-hydroxybutyrate) (OHB), oligo(3-hydroxybutyrate-co-4-hydroxybutyrate) (O3HB4HB) and oligo(3-hydroxybutyrate-co-3-hydroxyhexanoate) (OHBHHx) were used to treat cells. Murine beta cell line NIT-1 treated with OHBHHx displayed higher viability. Flow cytometric analysis of NIT-1 cells indicated that Oligo-HAs had an inhibitory effect on cell apoptosis. The cytosolic Ca2 + transient of NIT-1 cells increased when fed with 40 mg/L Oligo-HAs. Effect of OHBHHx was the best among all materials tested for gap junction intercellular communication of cells. Extracellular insulin secretion was up-regulated after growing in OHBHHx for 48 h. OHBHHx was not harmful to the beta cells [78].
3HB promoted proliferation of L929 cells, human umbilical vein endothelial cells, and rabbit articular cartilages [79], it had a stimulatory effect on DNA synthesis. 20 mg/L 3HB stimulated a rapid increase in the concentration of cytosolic calcium in L929 that was blocked by verapamil and diltiazem, inhibitors of L-type Ca2 + channels. These results indicated that 3HB had a stimulatory effect on cell cycle progression mediated by a signaling pathway dependent upon increases in an intracellular Ca2 + concentration. 3HB also promoted proliferation of L929 cells in high-density cultures by preventing apoptotic and necrotic cell death [80].
It was found that differentiation intensity of murine osteoblast MC3T3-E1 was in direct proportion to the 3HB concentration when it was lower than 10 mg/L. Calcium deposition, a strong indication of cell differentiation, demonstrated an obvious increase with increasing 3HB concentration from 0 to 100 mg/L. In vivostudy demonstrated that 3HB increased serum ALP activity and calcium deposition, leading to enhanced rat femur maximal load, bone deformation resistance and improved trabecular bone volume [81].
Percentages of mouse glial cells undergoing apoptosis decreased significantly in the presence of 3HB and its derivative 3-hydroxybutyrate methyl ester (HBME) [82]. In vitro study on cytosolic Ca2 + concentration revealed that 3HB and HBME dramatically elevated the cytosolic Ca2 + concentration. HBME worked more efficiently than 3HB did as HBME is more efficient in diffusing into the cells. 3HB and HBME demonstrated an inhibitory effect on cell apoptosis mediated by signaling pathways related to the elevation of cytosolic Ca2 + concentration. 3HB and HBME could become an effective neural protective agent.
3HB and HBME were found to significantly elevate neuroglial cell metabolic activity [83], due to 3HB receptor expressed in brain and upregulated in mice treated with HBME. HBME treatment was found to enhance expression of connexin 36 protein and phosphorylated ERK2 (extracellular signal-regulated kinase 2) in brain tissues. Mice treated with HBME behaved significantly better in the Morris water maze than either the negative controls (no treatment) or positive controls (acetyl-1-carnitine treatment). 3HB and HBME improved learning and memory, possibly attributing to a PUMA-G related signaling pathway requiring gap junctional intercellular communication [83].
5. Future prospective
As biodegradable plastics, PHA still have only a very limited market due to the high production cost [84], inconsistent properties as well as difficulties in plastic processing compared with mature conventional plastics from petroleum source [7], [85]. Therefore, two strategies have been adopted to meet this challenge: first, the development of a super PHA production strain combined with advanced fermentation processes to produce PHA at a low cost; second, the construction of functional PHA production strains with technology to control the precise structures of PHA molecules, this will allow the resulting PHA with high value added applications [86]. The excellent biodegradability and biocompatibility make PHA as outstanding medical and therapeutic materials.
Functionalized PHAs are obtained either by feeding structurally related substrates processed through the beta-oxidation pathway, or using specific strains able to transform sugars or glycerol into unsaturated PHA by de novofatty-acid biosynthesis [87]. In a UV mutant of Burkholderia sacchari, called LFM828, which transports hexoses and pentoses by a non-PTS uptake system, glutamate can also be used as carbon and energy allowing an improvement on the carbohydrates utilization for PHB production [88].
In vivo biodegradation of PHA is much slower compared with PLA and PLGA [89], [90], PHA are more suitable for applications requiring slower in vivodegradation [91]. To regulate biodegradation, blending of larger and smaller molecular weight PHA allows a reasonable mechanical strength with adjustable biodegradation [92]. Random- or block copolymerization or blending PHA with PLA or PLGA can help control biodegradation in vitro and in vivo.
So far only poly-4-hydroxybutyrate (P4HB) has been approved for as a suture material after more than 10 years clinical trial application. PHA available on the market including PHB, PHBV, P3HB4HB, PHBHHx, PHBVHHx and P3HB4HBHHx are targeting for low-end biodegradable packaging industry to avoid the lengthy and costly FDA medical approval. The targeted medical PHA should have a required stable composition, molecular weights and purity. So far, no medical standard has been developed for PHA therapeutic usages.
Among known therapeutic animal studies including drug delivery matrices, bone tissue engineering, artificial blood vessels, heart valve, artificial nerve conduits, lithography and injectable PHA [93], also some pharmaceutical applications (Table 1), 3D printing to generate specific shapes for personalized application looks most promising even though this is just starting to rise (Fig. 2). The PHA 3D printing market is large especially when considering the plastic surgery, traffic accidence demand that is increasing exponentially.
Table 1. The applications of PHA and its degradation products.
| PHA | Application | The relevant studies | References |
|---|---|---|---|
| PHB | Drug delivery systems | HepaRG cells were found to efficiently uptake PMLA-b-PHB-based nanoparticles. | [94] |
| PHA | Drug delivery matrices | PHA nanoparticles as biodegradable and biocompatible matrices for sustainable anticancer drug release. | [95] |
| PHB | Bone tissue engineering | PHB-PEG scaffolds better than PHB for growth of bone marrow stromal cells. | [96] |
| PHB/nHA | Bone tissue engineering | Human mesenchymal stromal cells (hMSCs) remained viable on PHB/nHA biocomposite scaffolds and proliferated continuously until reaching confluence. | [97] |
| PHBHHx | Artificial blood vessels and heart valve | Mechanical properties of the PHBHHx films were improved significantly by blending with PPC without affecting the biocompatibility, similar results achieved with mcl PHA. | [98], [99] |
| P4HB | Drug delivery, tissue engineering | P4HB should find use in a wide variety of medical fields such as cardiovascular, wound healing, orthopedic, drug delivery, and tissue engineering applications. | [100] |
| PHBHHx | Tissue transplantation | Islet cells on PHBHHx displayed higher metabolic activities than that on tissue culture plate, insulin gene expression and extracellular secretion was upregulated on PHBHHx for 72 h. | [101] |
| 3HB | Enzyme-protecting additive | 3HB could be used as an efficient enzyme-stabilizing and enzyme-protecting additive. | [102] |
| 3HD | Anti-cancer | Conjugation of anticancer DP18L peptide with 3HD enhances its anti-cancer activity; (R)-3-hydroxyalkanoic acids with 9 and 10 carbons were most effective at improving DPI8L activity. | [103] |
| HBME | Drug candidate | Mice treated with HBME performed significantly better in the Morris water maze than either the negative controls (no treatment) or positive controls (AXONA® treatment). It appeared that 3HB and HBME enhanced learning and memory of mice. HBME inhibited cell apoptosis under glucose deprivation, rescued activities of mitochondrial respiratory chain complexes that were impaired in AD (Alzheimer disease) patients and decreased the generation of ROS. | [104] |
| 3HB, HBME | Drug candidate | 3HB and its derivative HBME inhibited the development of osteoporosis in mice maintained under simulated microgravity, helping preserve bone microstructure and mechanical property. | [105] |
PMLA-b-PHB: poly(beta-malic acid) block poly(3-hydroxybutyrate) copolymer; hMSCs: human mesenchymal stem cells; PHB-PEG: poly(3-hydroxybutyrate)-poly(ethylene glycol) copolymer; 3HB: 3-hydroxybutyrate; 3HD: 3-hydroxydecanoic acid; HBME: 3-hydroxybutyrate methyl ester.